myosin molecules pulling actin filaments and myosin and actin are essentially nanomachines that convert the chemical energy of ATP hydrolysis - how cells in our muscles create energy - into mechanical work.
New electron cryomicroscopy images reveal unexpectedly large conformational changes in the myosin molecule during the pull and that may show how myosin generates force and create a paradigm for the construction of true nanomachines.
Myosin converts this energy by hydrolyzing ATP molecules into movement along an actin filament. The hydrolysis involves a number of conformational changes in myosin. These changes have been imaged using electron microscopy, but others didn't take atomic images of ATP hydrolysis when myosin is interacting with actin, which would more accurately represent the changes myosin takes during muscle contraction.

Architecture of skeletal muscle formed by regular arrays of myosin and actin filaments. Credit: Osaka University
Keiichi Namba, professor at Osaka University, and Takashii Fujii, assistant professor, used electron cryomicroscopy to acquire images at 5.2 Å resolution to show a previously unobserved conformational change in the myosin molecule when it interacts with actin. The two scientists hypothesized that this conformation could explain why muscle myosin has far faster kinetics than other myosin in the body.
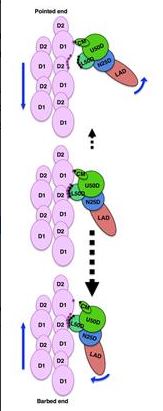 The images also give clues into how myosin moves along actin. The binding of ATP forces a rotation within the myosin molecule that reduces the number of bonds between it and actin. This weakly bound structure with a highly asymmetric bond distribution allows myosin to detach from actin and rebind preferentially in one direction, thus pulling the actin filament. Detachment toward the opposite direction would require a larger number of bonds to be broken at once, preventing detachment. This picture provides a new perspective on how myosin functions.
The images also give clues into how myosin moves along actin. The binding of ATP forces a rotation within the myosin molecule that reduces the number of bonds between it and actin. This weakly bound structure with a highly asymmetric bond distribution allows myosin to detach from actin and rebind preferentially in one direction, thus pulling the actin filament. Detachment toward the opposite direction would require a larger number of bonds to be broken at once, preventing detachment. This picture provides a new perspective on how myosin functions."This is a very unique image, because the weak binding state is unstable and it lifetime is short," noted Namba. He believes that this conformational state could be the reason why experiments on ATP hydrolysis by myosin without actin do not explain muscle contraction well.
"There is a structural asymmetry in the system", he said. "This could explain why myosin moves over much longer distance per ATP hydrolysis than expected."
Citation:Takashii Fujii and Keiichi Namba, Structure of actomyosinrigor complex at 5.2-Å resolution and insights into the ATPase cycle mechanism, Nature Communications, DOI: 10.1038/ncomms13969





Comments