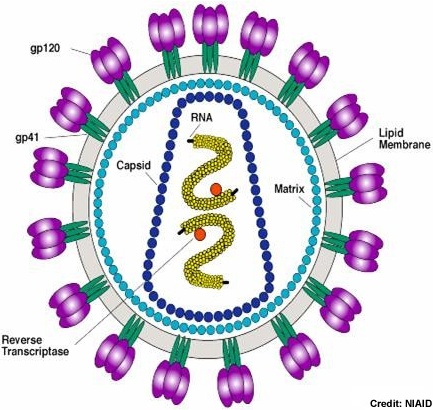
The HIV-1 virion uses the host cell membrane to form the viral envelope. Inside the lipid envelope, the matrix formed by Gag protein p17 holding the RNA containing core in place. The cylindrical core not only stores the viral RNA and various proteins, it also contains complementary RNA synthesized by the viral reverse transcriptase. Photo credit: Stanford University
Although countless hours of research and billions of dollars have been put into uncovering the key to developing a vaccine for HIV, we have only inched our way forward and are always uncovering a new hurdle to overcome before a viable vaccine is developed. The difficulties of making a vaccine effective against HIV arise at the structure of the virus. HIV translates itself through reverse transcriptase, an enzyme which can convert single stranded RNA into double stranded DNA. This mechanism is known as being “sloppy” at transcribing, making many mistakes and causing a fast rate of mutation, allowing the HIV virus to morph into infinite different strains as well as avoiding detection and treatment which targets the original form of the virus. A possible vaccine can only prevent one form of the disease, and is rendered useless by the mutation rate of HIV.
However new hope is on the horizon. Atlanta based biotechnology company, GeoVax, has developed a new version of a possible HIV vaccine and has launched their large scale Phase 2 clinical trials in 12 sites across North and South America. This clinical trial is being conducted in collaboration with the National Institutes of Health (NIH) and the HIV Vaccine Trials Network (HVTN).
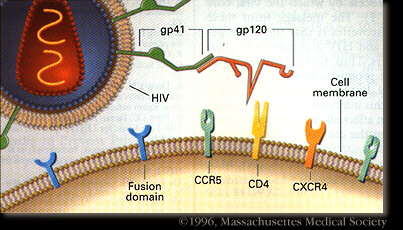
HIV-1 enters the blood stream most commonly through genital or colonic mucosa during sexual intercourse or other blood to blood contact. The virus enters the cell using the viral glycoprotein gp120 to interact with the host cellular recepter CD4 and chemockine receptor CCR5. The first cells targeted by the virus are usually the Langerhan cells, tissue dendritic cells of the lamina propia, and rarely direct infection of T cells. The main cellular targets of HIV-1 are the CD4+ T-helper/inducer subset of lymphocytes, CD4+ cells of macrophage lineage and dendritic cells. Photo credit: Stanford University.
The clinical trial will include a total of 225 participants, 150 in the vaccine group and 75 in the control group who will receive a placebo. The vaccine treatment includes a two part approach. There is a primer vaccine, which will “prime” the immune system, followed by a vaccine boost. Both parts express three major proteins of the HIV virus and comprise more than 50% of its makeup. The vaccine is comprised of HIV proteins, but cannot cause an HIV infection in the trial participants.
The core technology for the HIV/AIDS vaccine was developed through collaborative efforts among Emory University’s Vaccine Center, the NIH and CDC, as well as GeoVax Laboratories INC. Developed by Dr. Harriet Robinson at Emory University the HIV vaccine is recombinant DNA and MVA vaccine. The development of the AIDS vaccine is focused on the major HIV-1 subtypes (A,B and C), which cause a cellular and humoral immune reaction shown to have a 96% protection rate in the pre-clinical trials of non human primates.
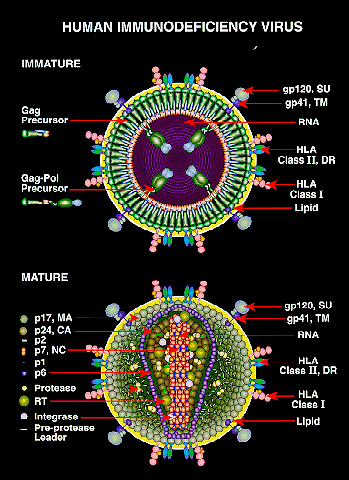
This image shows possible viral targets for treatment options or vaccines to prevent infection of HIV and its development into AIDS. Photo credit: NIAID Chemical Database-NIH
Instead of focusing primarily on creating antibodies to prevent HIV infection, this vaccine raises a cellular immune response in addition to raising antibodies. This double pronged approach has demonstrated a much higher efficacy in protecting against HIV infection than by either method alone. Cellular immunity is raised by T-cells in the body which destroy foreign proteins present in the body. The vaccine stimulates high T-cell activity and antibodies by combining DNA vaccine priming with a recombinant live virus MVA boost. This primer and boost method induces a protective immune response that will act to protect against an impending infection of HIV. Essentially this DNA/MVA vaccine stimulates both cellular and humoral immune responses. The cellular immune response generates certain types of immune system cells that recognize and respond to HIV. The humoral immune response produces antibodies that quickly respond to infection. Both are needed to establish long term protection against the development of AIDS.
This vaccine is designed to prevent the infection of HIV-1 in uninfected individuals and stopping the development of AIDS, and also offers another possible treatment option for those who have already been infected with HIV. If proven viable, this vaccine may provide us with a breakthrough in vaccine technology and development, and possibly bring to a halt one of the most devastating disease epidemics that humanity has ever seen.





Comments